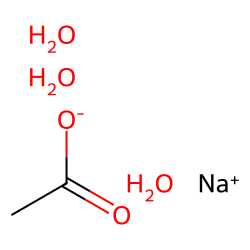
Temperature Dependent Properties
| Property | Value | Unit | Temperature (K) | Source |
|---|---|---|---|---|
| Cp,solid | [229.00; 322.00] | J/mol×K | [298.15; 325.00] | |
| Cp,solid | 229.00 | J/mol×K | 298.15 | NIST |
| Cp,solid | 322.00 | J/mol×K | 325.00 | NIST |
| ΔfusH | 20.25 | kJ/mol | 331.70 | NIST |
| ΔfusS | 61.00 | J/mol×K | 331.70 | NIST |
Similar Compounds
Find more compounds similar to Acetic acid, sodium salt, hydrate (1:1:3).
Mixtures
- Water + Acetic acid, sodium salt, hydrate (1:1:3)
- Xylose + Water + Acetic acid, sodium salt, hydrate (1:1:3)
- d-Arabinose + Water + Acetic acid, sodium salt, hydrate (1:1:3)
- D-Ribose + Water + Acetic acid, sodium salt, hydrate (1:1:3)
- L-Sorbose + Water + Acetic acid, sodium salt, hydrate (1:1:3)
- D-Fructose + Water + Acetic acid, sodium salt, hydrate (1:1:3)
- D-Galactose + Water + Acetic acid, sodium salt, hydrate (1:1:3)
- Glucose + Water + Acetic acid, sodium salt, hydrate (1:1:3)
- d-Mannose + Water + Acetic acid, sodium salt, hydrate (1:1:3)
- D-cellobiose + Water + Acetic acid, sodium salt, hydrate (1:1:3)
- Sucrose + Water + Acetic acid, sodium salt, hydrate (1:1:3)
- sodium chloride + Water + Acetic acid, sodium salt, hydrate (1:1:3)
Sources
- Effect of sodium acetate on the volumetric behaviour of some mono-, di-, and tri-saccharides in aqueous solutions over temperature range (288.15 to 318.15) K
- Equilibria in the Ternary System NaCl NaAc H2O at 303.15 K, 323.15 K, and 343.15 K
- NIST Webbook
Note: Cheméo is only indexing the data, follow the source links to retrieve the latest data. The source is also providing more information like the publication year, authors and more. Take the time to validate and double check the source of the data.